Chronic Kidney Disease
Chronic kidney disease (CKD) is a progressive disease affecting kidney function that occurs over months to years. It may present with:
- Persistent proteinuria or abnormal renal morphology
- Hypertension in most cases
- Uremia when nearing end-stage disease (ESRF)
- Bilateral small or echogenic kidneys upon ultrasound is found in advanced disease.
CKD is defined as either kidney damage or GFR < 60 mL/min/1.73 m² for 3 or more months
- “Kidney damage” is defined as pathologic abnormalities or markers of damage (abnormalities in blood, urine, or imaging studies)
- Glomerular Filtration Rate (GFR) is a primary method for staging CKD
Stage Description GFR (mL/min/1.73 m²) 1 Kidney damage with normal or elevated GFR ≥90 2 Kidney damage with mildly decreased GFR 60 — 89 3a Mildly to moderately decreased GFR 45 – 59 3b Moderately to severely decreased GFR 30 – 44 4 Severely decreased GFR 15 – 29 5 End-stage Kidney Disease <15 (or dialysis)
Etiology
- Tubulointerstitial nephritis: drug hypersensitivity, pyelonephritis, sickle cell nephropathy, idiopathy
- Cystic diseases
- Obstructive nephropathies: stone formation, tumor
- Glomerular diseases: diabetic nephropathy, infections
- Vascular diseases: hypertension
Risk Factors for Faster Progression
The rate of kidney deterioration in CKD may accelerate with the following:
- Increasing proteinuria (risk begins with 30 mg/day, most risk with >1000 mg/day)
- Higher blood pressure
- Decreased HDLs
- Smoking and alcohol use
- Poor control of diabetes mellitus
- Use of NSAIDs
- Obesity
- Race, old age, primary kidney disease (non-modifiables)
Pathophysiology
- CKD leads to progressive decline in RF even after the removal of an inciting cause. This mechanism primary involves the loss of nephrons, the functional unit of the kidneys.
- Destruction of nephrons leads to compensatory hypertrophy and supranormal GFR in remaining nephrons in an attempt to maintain homeostasis.
- Compensatory hyperfiltration leads to overwork injury, which progressively produces glomerular sclerosis and interstitial fibrosis.
- Consequently,
- Nitrogenous waste products (urea) are retained, and produce uremic syndrome
- Metabolic and endocrine kidney functions fail, which produce anemia, metabolic bone disorders, among other manifestations.
Assessment Findings
CKD is insidious; no symptoms may appear until GFR is markedly deficient.
- The most common clinical finding is hypertension
- Edema, discolored urine, flank pain
- Increased drug toxicity for medications excreted by the kidney (e.g., insulin → hypoglycemia, especially as DM is a major etiology for CKD)
- Decreased libido, menstrual irregularities: hormonal imbalances from renal dysfunction
- Uremic Syndrome: symptoms produced by the build-up of urea (creatinine, BUN) in the blood known as azotemia. This manifests:
- Fatigue: anemia, toxin build-up
- Anorexia, Nausea: waste-product build-up
- Metallic mouth taste, halitosis: urea may be broken down into ammonia in saliva
- Generalized pruritus with no rash: phosphate buildup and toxin accumulation
- Pericarditis with pleuritic chest pain; “uremic pericarditis” as uremic toxins irritate the pericardial lining
- Uremic Encephalopathy: decreased mental status, asterixis (flapping tremors), myoclonus, possible seizures. Additionally,
- Memory impairment
- Insomnia
- Restless legs
- Twitching
Screening and Early Detection
CKD is insidious, and may be advanced when discovered. Screening is useful because interventions may be effective in slowing disease progression. While mass screening is not recommended, those with the following risk factors (high risk group) should be checked:
- Diabetes Mellitus, Hypertension
- Those who recovered from Acute Kidney Injury
- Urolithiasis or history of urinary tract obstruction
- Family history of CKD
- Infections: systemic, UTI, HIV
- Neoplasia, Autoimmune Disease
- Those using Nephrotoxic Drugs or are hospitalized
Spot urinalysis:
- Check urine albumin (normally <20 mg/day). Persistent excretion of >300 mg/day or an albumin-to-creatinine ratio of >300 mg/g is considered albuminuria.
Diagnostic Evaluation
- Serum creatinine can be used to estimate GFR.
- Urine RBC, WBC
- Serum electrolytes (Na, K, Cl, HCO₃)
A kidney ultrasound scan can be used to assess kidney size, echogenicity, corticomedullary differentiation, evidence of obstruction.
- Bilaterally small, echogenic kidneys suggest chronic scarring found in advanced CKD.
- Large kidneys may be noted in adults with adult polycystic kidney disease, diabetic nephropathy, HIV-associated nephropathy, plasma cell myeloma, amyloidosis, and obstructive uropathy.
Complications
- Cardiovascular Complications: hypertension, coronary artery disease, heart failure, atrial fibrillation, pericarditis
- Metabolic Bone Disease: an imbalance of calcium, phosphate, PTH, and vitamin D from kidney disease stimulate bone resorption. This is the second earliest complication to appear, following hypertension.
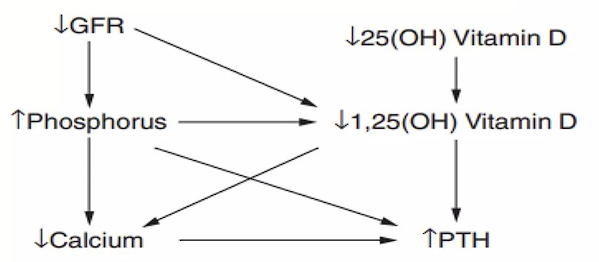
- Hematologic Complications: anemia (normocytic; erythropoietin deficiency), coagulopathy
- Hyperkalemia
- Acid-base Disorders: the kidneys are a major regulator for acid-base balance.
- Neurologic Complications
- Endocrine Disorders: from failure to excrete hormones
Management
- Evaluation: determine the type of kidney disease, comorbid conditions, current level of kidney function, complications present related to kidney function, and further risks for kidney or cardiovascular disease.
- Treatment of reversible causes for renal dysfunction. Therapy is dependent on diagnosis and comorbid conditions.
- Prevention of progression: it is vital to treat the underlying cause. Some examples of causes include are:
- Renal hypoperfusion: hypovolemia, hypotension, infection, and drugs that lower GFR (NSAIDs)
- Cease nephrotoxic drug use: long-term NSAID use
- Treat UTIs
- Treat UT obstruction
- Strict control of diabetes mellitus and other comorbid conditions
- Blood pressure control
- Weight loss if obese
- Treat metabolic acidosis
- SGLT2 can be used to slow progression
- Treatment and prevention for complications
- Hypertension: target a blood pressure of 130/80-85; if proteinuria or diabetes mellitus is present, then 120/80 is the target. Use diuretics, sodium restriction, and antihypertensives (ACE inhibitors, Angiotensin receptor blockers, calcium channel blockers)
- Diabetes Mellitus: target a strict control of serum glucose to 100–140 (bedtime) and 80–120 (prepranial) mg/dL. A HbA1c should also be kept <7%
- Anemia: target a hemoglobin level of 10 to 13 g/dL. Epogen, iron, and folic acid may be used.
- Hypolipidemics may be used to control dyslipidemia.
- Identification of indications for and adequate preparation for renal replacement therapy (RRT; dialysis, transplantation) is done if uremia is present.
- Diet Regimen:
- Restrict protein: use a plant-based diet
- Restrict salt and water: 2 g of salt, 2 L of fluids
- Restrict potassium: hyperkalemia may occur as the kidneys fail to excrete them; additionally, potassium-binding resins may be passed through the bowel to reduce potassium levels.
- Restrict phosphorus.
- Medications: the kidneys are responsible for the excretion of many drugs and their metabolites. Some drugs which should be adjusted in dosage or discontinued entirely are:
- Insulin can produce hypoglycemia when the kidney fails to remove insulin from the bloodstream.
- Metformin accumulation results in lactic acidosis.
- Morphine metabolites are removed by the kidneys. Prolonged sedation can occur in renal impairment.
- Nephrotoxic drugs: NSAIDs and contrast material reduce renal blood flow, which can produce acute kidney injury from nephropathy.
- Magnesium containing laxatives: hypermagnesemia
- Phosphorus containing drugs (e.g. cathartics): hyperphosphatemia
End-Stage Kidney Disease
In ESKD, renal replacement therapy is absolutely necessary. An early referral to a nephrologist when reaching late stage 3 CKD or a rapidly declining GFR is imperative for good prognosis.
- Utilize a team approach: collaborate with dieticians, nephrologists, etc.
- Educate the patient about the procedure and its pertinent information.
- Palliate the client while awaiting or undergoing RRT.
Dialysis
Dialysis is done when GFR is down to 10 mL/min/1.73 m², when uremic symptoms appear, when fluid overload persists despite diuresis, and when refractory hyperkalemia occurs. This may take the form of:
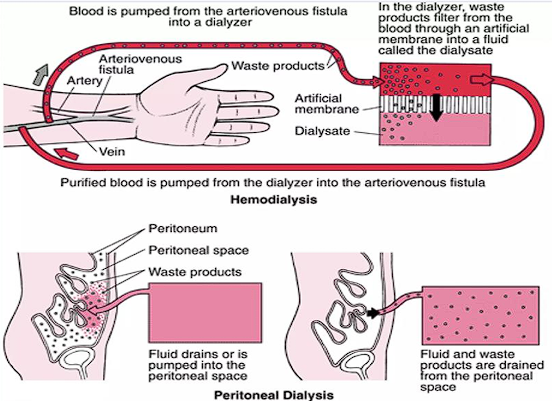
Hemodialysis
The blood is physically removed from the body, moved through a dialyzer (filters the waste), then returned as purified blood into the body. This is done thrice a week for 3 to 5 hours when at a dialysis center, or done at home (more frequent, shorter length)
- This often utilizes an arteriovenous (AV) fistula which requires special precautions for trauma to the arm with the fistula. Alternatively, an indwelling catheter may be used. It’s not without complications, however— infections (often staphylococcus), thrombosis, and aneurysms may occur.
- Complications include fever, hypotension (excessive filtration, hemorrhage, etc.), and issues brought about by failure of dialysis: hemolysis (chloramine from dialysate), dementia (aluminum from dialysate), seizures (failure of dialysis concentrate delivery system), bleeding (excessive anticoagulants), and muscle cramps (from ultrafiltration)
Peritoneal Dialysis
A dialysis solution is pumped into the peritoneum, where the peritoneal membrane acts as a semipermeable membrane to excrete waste products. The dialysis solution is drained and cycled.
- This may be continuous ambulatory peritoneal dialysis (CAPD) or continuous cyclic peritoneal dialysis (CCPD). A machine is required for CCPD.
- Peritonitis is a major concern. Nausea, vomiting, abdominal pain, diarrhea or constipation, and fever may appear. The clear dialysate becomes cloudy, and peritoneal cell count increases to 100 WBC/mcl. Staph A is the most common causative agent, but strep. and G-ves may also be causative. Antibiotics (emepric intraperitoneal vancomycin or 1st gen cephalosporins, + 3rd gen cephalosporin [Ceftazidime], then abx rx later tailored to culture results) i just copied this directly may be used to treat peritonitis.
- Other complications include exit-site or tunnel infections, and noninfectious complications such as catheter issues (impaired flow, leakage, pain), increased abdominal pressure (backpain, hernia, hydrothorax), metabolic issues (hypokalemia, metabolic syndrome), and encapsulating peritoneal sclerosis.
Kidney Transplantation
An issue of supply is always present in transplants. Patients can wait years to receive a fitting donor. Two-thirds of kidney allografts come from deceased owners, with the remaining coming from living donors.
Patients undergoing dialysis have an average 3 to 5 year life expectancy (can go up to 25 years, depending on comorbidities), but the average waiting time (in the U.S.) is 3 to 7 years. The most common (majority) cause of death is cardiac disease, with other deaths appearing from infection, cerebrovascular disease, or malignancy.
A patient is referred as a candidate for transplantation by a nephrologist once they reach stage 3 to 5 of CKD, CKD with polycystic kidney disease, or those with proteinuria of more then 1000 mg/day. It is important to refer early especially for those with rapidly progressing decline in renal function, even if the aforementioned criteria have not been met.
When to Admit
CKD decompensation (acid-base and electrolyte balance disorder worsens, volume overload reappears) and those starting dialysis are to be admitted to a care institution.