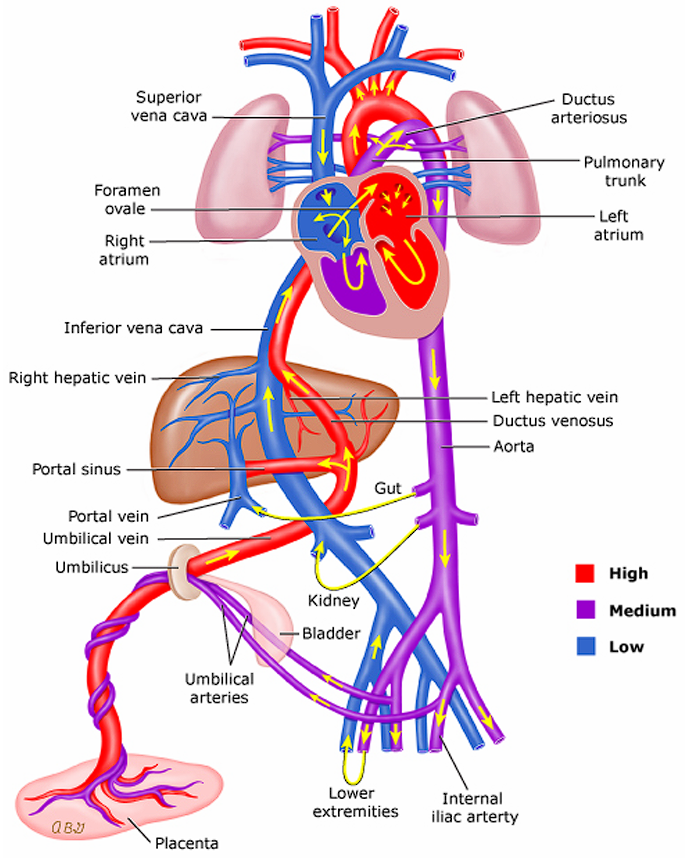
Fetal Circulation
As a review, there are two external and three internal parts of fetal circulation that allows the fetus to oxygenate in-utero. These are the placenta, umbilical cord, ductus arteriosus, ductus venosus, and foramen ovale.
- Oxygenated blood from the placenta is sent through the umbilical vein through the ductus venosus and directly into the inferior vena cava. This bypasses the normal adult circulation through the liver. A minute amount of blood is still passed through the liver for nourishment. The inferior vena cava (oxygenated) and superior vena cava (deoxygenated) deposit blood into the right atrium.
- Blood coming in from the inferior vena cava will mostly pass through the foramen ovale into the left atrium, as it closes the superior vena cava and tricuspid valve. From the left atrium, blood is pumped to the left ventricle and through the aorta, which nourishes the brain.
- Blood coming in from the superior vena cava will mostly pass through the tricuspid valve into the right ventricle, through the pulmonary artery, through the ductus arteriosus, and to the descending aorta.
- Deoxygenated blood is sent back to the placenta through the umbilical arteries from two arteries past the bifurcation of the descending aorta.
- The ductus (“ducts”; temporary connections between two vessels) shunts blood in fetal circulation. There are three shunts in a fetus:
- Ductus venosus: connects the umbilical vein and inferior vena cava, bypassing the immature liver.
- Ductus arteriosus: connects the pulmonary artery and aorta, allowing blood to shunt from right-to-left.
- Foramen ovale: connects the two atria, allowing blood to shunt left-to-right, bypassing the lungs.
- After clamping and cutting and at delivery, fetal circulation begins to adapt and become alike to adult circulation.
- The umbilical vessels (arteries and vein) will shrink and close.
- The baby’s lungs expand, which begin to receive more blood. This blood returns to the left atrium of the heart, increasing left-side pressure (and decreasing right-side pressure), and closing the flap of the foramen ovale. This occurs within 12 hours.
- Decreasing prostaglandin will also result in the closure of the ductus arteriosus as oxygen concentration increases, constricting the duct. This occurs within 24 hours.
- The longest waiting time is 48 hours for the foramen ovale and ductus arteriosus to close before pathology is suspected. In assessment, these manifest as murmurs.
Tip
Absolutely do memorize the normal pathway of blood through the body and the heart. Visualization of how blood moves across each chamber and major vessel in each defect is very important in understanding the nature of the congenital heart defects:
- IVC, SVC, and Coronary Vessels deposit blood into the Right Atrium
- Right Atrium, Tricuspid Valve, Right Ventricle
- Right Ventricle, Pulmonary Valve, Pulmonary Artery, Lungs
- Lungs, Pulmonary Veins, Left Atrium
- Left Atrium, Mitral/Bicuspid Valve, Left Ventricle
- Left Ventricle, Aortic Valve, Aorta
Congenital Heart Defects
The main cause of CHDs is unknown. However, infants can be predisposed to CHDs in alcoholism, maternal malnutrition, rubella, teratogenesis (drugs, radiation), advanced maternal age, and family history of CHDs.
Rubella
German measles is highly teratogenic, especially during the first trimester. Even a simple exposure is enough to cause damage, even if the mother does not experience any symptoms.
Diagnostic Examination
- History, Physical Examination
- Chest X-ray
- ECG
- CBC, ABG
- Angiogram
- 2D Echo (Ultrasound of the heart)
- Cardiac Catheterization
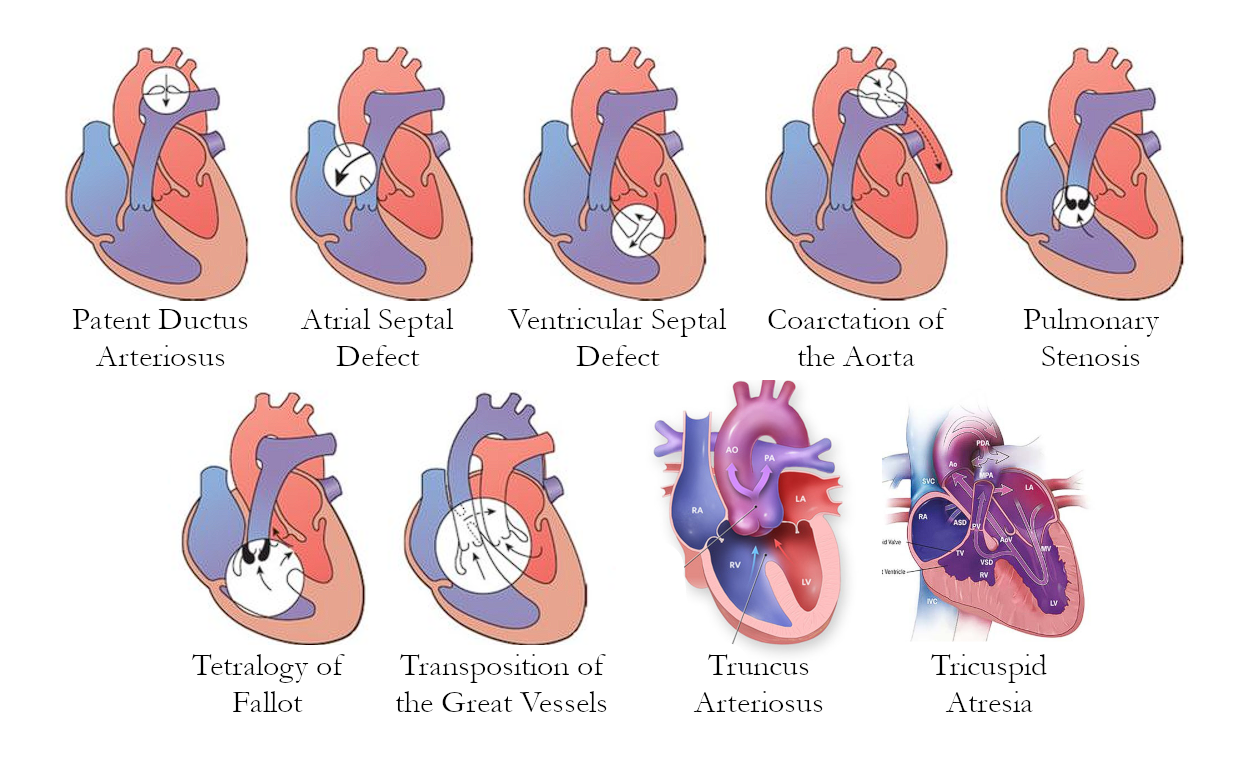
Diagrams
I still don’t like how much space images take, so I’ve merged them all into one image.
Acyanotic CHDs
Heart defects that do not produce cyanosis. These may be due to obstructive lesions such as stenoses and coarctation, or due to left-to-right shunting. These send oxygenated blood back into pulmonary circulation, which means no significant deoxygenation occurs.
Valvular Stenosis
Valvular Stenosis (obstructive): restriction/narrowing of the pulmonary or aortic valve, limiting blood flow. This reduces cardiac output, but does not send deoxygenated blood into systemic circulation.
- Restricted blood flow will result in ventricular hypertrophy. Right-sided if pulmonic, and left-sided if aortic.
- Pulmonary Valve Stenosis will cause a backup of blood back into the right atrium and inferior vena cava. This is a form of right-sided congestive heart failure.
- Aortic Valve Stenosis will cause a backup of blood back into the left atrium and pulmonary circulation. This is a form of left-sided congestive heart failure.
- Correction will require valvuloplasty or valve replacement.
Coarctation of the Aorta
Coarctation of the Aorta (obstructive): the descending aorta is narrow, limiting blood flow to the lower extremities. The primary presenting manifestation of this disorder is hypertension of the upper extremities, and hypotension of the lower extremities. Pulse, temperature, and color also differ between the upper and lower extremities.
- Resection and end-to-end anastomosis is used to remove the section of the aorta that is narrowed. A bypass is used while the aorta is being repaired.
- Constipation must be avoided to prevent straining, to avoid damage to the suture.
Patent Ductus Arteriosus
Patent Ductus Arteriosus (LTR shunt): a duct connecting the aorta and pulmonary artery allows oxygenated blood to enter pulmonic circulation. This normally closes within 24 hours as prostaglandin levels go down, but may remain open. Indomethacin can be given to stimulate the closure of the duct, and prostaglandin may be used to keep it open. If ineffective, surgery: ligation and cauterization may be used.
- The murmur produced by a PDA is a machinery-like murmur.
Atrial Septal Defect
Atrial Septal Defect (LTR shunt): the septal wall of the atria is open, allowing blood to flow from the left atrium to the right atrium. Oxygenated blood pumps back into the lungs, increasing pulmonary flow. Congestion of the right atrium may also result in right-sided CHF.
- A Dacron/Telfon Septal Patch is placed via open heart surgery to correct the defect.
Ventricular Septal Defect
Ventricular Septal Defect (LTR shunt): the septal wall of the ventricles is open, allowing blood to flow from the left ventricle to the right ventricle. This is the most common congenital heart defect. Oxygenated blood is pumped back into the lungs, increasing pulmonary flow. Congestion of the right ventricle may also result in right-sided ventricular hypertrophy. - In compensating for the congestion, the right ventricle may overpower the left ventricle, and reverse the shunt from LTR to RTL. - The murmur produced by a VSD is an abnormally loud murmur. - A Dacron/Teflon Septal Patch is placed via open heart surgery to correct the defect.
Combination!
Atrioventriculocanal Defect (LTR shunt): the presence of both atrial and ventricular septal defects, combined into one large opening, or two separate openings. This further increases right-sided congestion. Patching is still the method for correction.
Cyanotic CHDs
The only type of CHDs that appear cyanotic are those with right-to-left shunting where deoxygenated blood flows into systemic circulation and pulmonary circulation is decreased. (These all start with T)
Tetralogy of Fallot
Tetralogy of Fallot (TOF): a combination of four defects (mn. PROVe) that appears as the most common cyanotic heart defect:
- Pulmonary Valve Stenosis restricts pulmonary blood flow.
- Right Ventricular Hypertrophy is caused by the ventricular septal defect and pulmonary stenosis. This increases the contractility of the right ventricle, which may reverse flow to right-to-left shunting as it overpowers the left ventricle, accessing the aorta. This creates the boot-shaped heart observed during a 2D ECHO.
- The Overriding of the aorta is where the aorta is set between the articles and as such is accessible by both the left and right ventricle; it receives blood from both ventricles.
- A Ventricular Septal Defect allows for blood to flow from the right ventricle into the aorta.
Pathophysiology: hypoxia results in tachycardia, tachypnea, polycythemia (hemoconcentration, increased coagulability), small-for-gestational-age, and prematurity.
- Failure to oxygenate properly will impair feeding. The infant will instinctively attempt to breathe when feeding, and this may result in aspiration. Keep the infant upright during feedings.
- CHDs generally increase the risk for infection: Pneumonia, otitis media, and infective endocarditis are common complications, particularly from S. aureus and GAHBS.
Management: the presence of chronic hypoxia will result in clubbing, and crying or feeding may result in a Tet. spell, a hypercyanotic or “choking” spell/state. The Squatting/Knee-chest Position will improve oxygenation, and may be observed or used after exertion.
- The formula used for feeding the infant will be Lonalac, a low-sodium formula to reduce fluid retention (edema) from congestive heart failure secondary to TOF.
- To prevent developmental delays, allow the child to moderate their activities and exertion after educating both the child and parents in how to manage cyanotic spells.
Treatment:
- Palliative care uses a Blalock-Taussig Shunt. This procedure connects the two subclavian arteries to the two pulmonary arteries to increase pulmonary blood flow and oxygenation.
- Pharmacologic: an inotrope, Digitalis (Lanoxin), is used to improve heart contractility in congestive heart failure. Always check for bradycardia (<100 in infants) before administration as inotropes have a negative chronotropic (lowers heart rate) effect.
- Correction: relieving pulmonary stenosis through valvuloplasty or valve replacement, the overriding of the aorta, then the ventricular septal defect. These procedures are done with three open heart surgeries done in stages.
Transposition of the Great Vessels
Transposition of the Great Arteries/Vessels: swapping of the connections of the pulmonary artery and aorta. This shuts off pulmonary circulation from systemic circulation as the right ventricle sends blood into systemic circulation and the left ventricle sends blood into the lungs. Survival rates for TOGV are very poor.
- The only way a newborn can survive with this defect is with the presence of a foramen ovale and patent ductus arteriosus. Oxygenated blood may move from the pulmonary artery (now connected to the left ventricle) into the aorta through the PDA, and a left-to-right shunt through the foramen ovale allows oxygenated blood to move from the left atrium to the right atrium.
- This defect is visible in a 3D ECHO.
- Treatment:
- Palliative: Prostaglandin is used to keep the ductus arteriosus open and the Rashkind procedure is used to create an atrial septal opening.
- Corrective: the Mustard procedure is used to redirect blood flow. The atrial septal opening is also closed.
Truncus Arteriosus
Truncus Arteriosus: the aorta and pulmonary artery fail to separate, and create one large vessel that takes in blood from both the left and right ventricles through a large ventricular septal defect.
- Treatment: Complete cardiac repair: division of the conjoined pulmonary artery and aorta, and closure of the large ventricular septal defect.
Tricuspid Atresia
Tricuspid Atresia: failure of the tricuspid valve to form. This isolates the right ventricle from the right atrium. The only method by which a newborn is able to survive with this defect is with the presence of the foramen ovale or an atrial septal defect and a ventricular septal defect, allowing the blood to move from the right atrium to the left atrium, to the left ventricle, then into the right ventricle to send blood into the lungs.
- Treatment:
- Palliative: Blalock-Taussig Shunt (see Tetralogy of Fallot) and a Waterston Shunt, an anastomosis of the ascending aorta and right pulmonary artery to increase pulmonary blood flow.
- Corrective: the Fontan Procedure that consists of (1) placement of a conduit with a valve between the right atrium and pulmonary artery, (2) closure of the ASD, and (3) ligation just above the pulmonary valve of the main pulmonary artery, as this is no longer the entry point used. The VSD may also be closed.
Summary
| Congenital Heart Defect | Description | Treatment |
|---|---|---|
| Patent Ductus Arteriosus | The ductus arteriosus fails to close within 48 hours (normally 12 hours) | Indomethacin or Ligation if ineffective |
| Atrial Septal Defect | An opening between the atria | Patching |
| Ventricular Septal Defect | An opening between the ventricles | Patching |
| Atrioventriculocanal Defect | A combination of ASD and VSD | Patching |
| Pulmonary Valve Stenosis | Narrowing of the pulmonary valve | Valvuloplasty/Valve Replacement |
| Aortic Valve Stenosis | Narrowing of the aorta | Valvuloplasty/Valve Replacement |
| Tetralogy of Fallot | Pulmonary valve stenosis, right-sided ventricular hypertrophy, overriding of the aorta, and a ventricular septal defect | Blalock-Taussig; Correction of stenosis, then overriding, then the septal defect |
| Transposition of the Great Vessels | Swapping of the pulmonary artery and aorta, separating pulmonic circulation from systemic circulation | Prostaglandin, Rashkind Mustard |
| Truncus Arteriosus | Conjoining of the pulmonary artery and aorta with a large ventricular septal defect | Complete cardiac repair |
| Tricuspid Atresia | Failure of the tricuspid valve to form or open, requiring an atrial septal defect and ventricular septal defect to maintain circulation | Blalock-Taussig, Waterston Fontan |